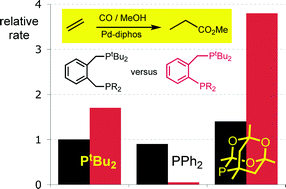
The following unsymmetrical diphosphines have been prepared: o-C6H4(CH2PtBu2)(PR2) where R = PtBu2 (L3a3a3a); PCg (L3b3b); PPh2 (L3c3c3c); P(o-C6H4CH3)2 (L3d3d3d); P(o-C6H4OCH3)2 (L3e3e) and o-C6H4(CH2PCg)(PCg) (L3f3f) where PCg is 6-phospha-2,4,8-trioxa-1,3,5,7-tetramethyladamant-6-yl. Hydromethoxycarbonylation of ethene under commercially relevant conditions has been investigated in the presence of Pd complexes of each of the ligands L3a–f3a–f and the results compared with those obtained with the commercially used o-C6H4(CH2PtBu2)2 (L1a1a). The Pd complexes of the bulkiest ligands L3a3a3a, L3b3b and L3f3f are highly active catalysts but the Pd complexes of L3c3c3c, L3d3d3d and L3e3e are completely inactive. The crystal structures of the complexes [PtCl2(L1a1a)] (1a) and [PtCl2(L3a3a3a)] (2a) have been determined and show that the crystallographic bite angles and cone angles are greater for L1a1a than L3a3a3a. Solution NMR studies show that the seven-membered chelate in 1a is more rigid than the six-membered chelate in 2a. Treatment of [PtCl(CH3)(cod)] with L3a–f3a–f gave [PtCl(CH3)(L3a–f3a–f)] as mixtures of 2 isomers 3a–f and 4a–f. The ratio of the products 4 : 3 ranges from 100 : 1 to 1 : 20, the precise proportion is apparently governed by a balance of two competing factors, steric bulk and the antisymbiotic effect. The palladium complexes [PdCl(CH3)(L3b3b)] (5b/6b) and [PdCl(CH3)(L3c3c3c)] (5c/6c) react with labelled 13CO to give the corresponding acyl species [PdCl(13COCH3)(L3b3b)] (7b/8b) and [PdCl(13COCH3)(L3c3c3c)] (7c/8c). Treatment of [PdCl(13COCH3)(L)] with MeOH gave CH313COOMe rapidly when L = L3b3b but very slowly when L = L3c3c3c paralleling the contrasting catalytic activity of the Pd complexes of these two ligands.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...