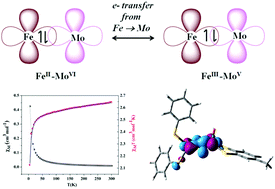
Mo–Fe heterometallic complexes with Fe(X)2 (X = Cl, SPh) moiety attached to monodithiolene oxomolybdenum via sulfur bridge, viz., [Ph4P]2[Cl2FeS2MoOS2(DMED)] (2) (DMED, dimethylethylenedicarboxylate), [Ph4P]2[Cl2FeS2MoO(tdt)] (3) (tdt, toluenedithiolate) and [Ph4P]2[(SPh)2FeS2MoO(tdt)] (4) are reported. Mossbauer spectroscopy, magnetism, EPR, electrochemistry and electronic structure based on DFT and TD-DFT calculation show the transfer of electron from iron to molybdenum centre resulting antiferromagnetically coupled Fe(III)Mo(V) unit from the starting Fe(II) and Mo(VI) compounds. A net spin of S = 2 ground state arising from antiferromagnetically coupled Fe(III) and Mo(V) shows a rare X-band EPR in normal mode at g ∼ 12 in the solid state. In addition, Mossbauer studies show that electron drifting is more pronounced upon substitution of the chloride ligand by thiophenolate. The changes in dithiolene periphery electronically affect the charge distribution between Mo–Fe in {OMo(μS)2Fe} core. DFT calculations indicate that the increasing stability of dative Fe → Mo hetero metal–metal bond in these complexes from 3 to 2 to 4 is related to the extent of electron transfer from the iron to molybdenum centre.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...