Synthesis, X-ray characterization and computational studies of Cu(ii) complexes of N-pyrazolyl pyrimidine†
Abstract
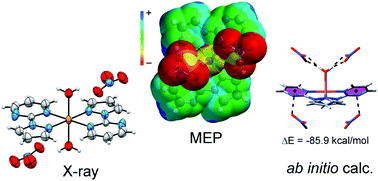
In this manuscript we report the synthesis and X-ray characterization of several complexes of Cu(II) with a 2-(1H-pyrazol-1-yl)-pyrimidine (L)

 Please wait while we load your content...
Please wait while we load your content...