Hydrogenolysis reactions of so-called lignin model dimers using a Ru-xantphos catalyst are presented (xantphos = 4,5-bis(diphenylphosphino)-9,9-dimethylxanthene). For example, of some nine models studied, the alcohol, 2-(2-methoxyphenoxy)-1-phenylethanol (1), with 5 mol% Ru(H)2(CO)(PPh3)(xantphos) (18) in toluene-d8 at 135 °C for 20 h under N2, gives in ∼95% yield the C–O cleavage hydrogenolysis products, acetophenone (14) and guaiacol (17), and a small amount (<5%) of the ketone, 2-(2-methoxyphenoxy)-1-phenylethanone (4), as observed by 1H NMR spectroscopy. The in situ Ru(H)2(CO)(PPh3)3/xantphos system gives similar findings, confirming a recent report (J. M. Nichols et al., J. Am. Chem. Soc., 2010, 132, 12554). The active catalyst is formulated ‘for convenience’ as ‘Ru(CO)(xantphos)’. The hydrogenolysis mechanism proceeds by initial dehydrogenation to give the ketone 4, which then undergoes hydrogenolysis of the C–O bond to give 14 and 17. Hydrogenolysis of 4 to 14 and 17 also occurs using the Ru catalyst under 1 atm H2; in contrast, use of 3-hydroxy-2-(2-methoxyphenoxy)-1-phenyl-1-propanone (7), for example, where the CH2 of 4 has been changed to CHCH2OH, gives a low yield (≤15%) of hydrogenolysis products. Similarly, the diol substrate, 2-(2-methoxyphenoxy)-1-phenyl-1,3-propanediol (9), gives low yields of hydrogenolysis products. These low yields are due to formation of the catalytically inactive complexes Ru(CO)(xantphos)[C(O)C(OC6H4OMe)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(Ph)O] (20) and/or Ru(CO)(xantphos)[C(O)CH
C(Ph)O] (20) and/or Ru(CO)(xantphos)[C(O)CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(Ph)O] (21), where the organic fragments result from dehydrogenation of CH2OH moieties in 7 and 9. Trace amounts of Ru(CO)(xantphos)(OC6H4O), a catecholate complex, are isolated from the reaction of 18 with 1. Improved syntheses of 18 and lignin models are also presented.
C(Ph)O] (21), where the organic fragments result from dehydrogenation of CH2OH moieties in 7 and 9. Trace amounts of Ru(CO)(xantphos)(OC6H4O), a catecholate complex, are isolated from the reaction of 18 with 1. Improved syntheses of 18 and lignin models are also presented.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(Ph)O] (20) and/or Ru(CO)(xantphos)[C(O)CH
C(Ph)O] (20) and/or Ru(CO)(xantphos)[C(O)CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(Ph)O] (21), where the organic fragments result from
C(Ph)O] (21), where the organic fragments result from 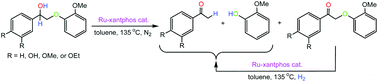

 Please wait while we load your content...
Please wait while we load your content...