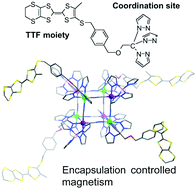
New TTF-based (TTF = tetrathiafulvalene) ligands, L1 and L2 (L1 = α-(4′-methyl-4,5-ethylenedithiotetrathiafulvalene-5′-thio)-α′-[tris-2,2,2-(1-pyrazolyl)ethoxy]-p-xylene and L2 = α-(4′-methyl-4,5-dimethylthiotetrathiafulvalene-5′-thio)-α′-[tris-2,2,2-(1-pyrazolyl)ethoxy]-p-xylene), possessing tris-pyrazolyl coordination sites, were synthesized. The reactions of Ni(BF4)2·6H2O with the TTF-ligands (L1 and L2), n-Bu4N[Fe(CN)3(tp or pztp)] (tp = hydrotris(pyrazol-1-yl)borate and pztp = tetrakis(pyrazol-1-yl)borate) in the presence of additional counter ions afforded two cyanide-bridged octanuclear complexes: [FeIII4NiII4(CN)12(pztp)4(L1)4](BF4)4 (1) and [FeIII4NiII4(CN)12(pztp)4(L2)4](PF6)4 (2). Using a similar procedure to that employed in the synthesis of complex 2, with the addition of sodium tetraphenylborate, yielded a two-electron-reduced compound, Na[FeIII2FeII2NiII4(CN)12(tp)4(L2)4](BF4)3 (3), in which a sodium ion was encapsulated by the cube. The host–guest complex 3 showed enhanced redox behaviour and while magnetic susceptibility measurements revealed ferromagnetic interactions to be operative in all three complexes, the cation encapsulation behaviour of 3 led it to exhibit single molecule magnet-type properties.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...