Unusual oxidation state distributions observed for two mixed-valence heptanuclear manganese disc-like clusters†
Abstract
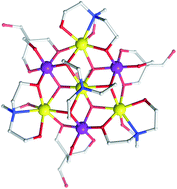
The synthesis, structures and magnetic properties of two new mixed-valence heptanuclear manganese clusters are described. Both complexes utilize

 Please wait while we load your content...
Please wait while we load your content...