η1-Allylpalladium complexes with a tridentate PNP ligand with different phosphino groups†
Abstract
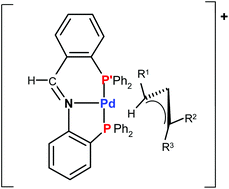
The iminodiphosphine 2-(PPh2)C6H4-1-CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NC6H4-2-(PPh2) (P–N–P′) is used for the preparation of the complexes [Pd(η1-CHR1–CH
NC6H4-2-(PPh2) (P–N–P′) is used for the preparation of the complexes [Pd(η1-CHR1–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CR2R3)(P–N–P′)]BF4 [R1 = R2 = R3 = H: (1); R1 = R2 = Ph, R3 = H: (2); R1 = R3 = H, R2 = Ph: (3); R1 = H, R2 = R3 = Me: (4)]. The P–N–P′ tridentate coordination and the η1-allyl bonding mode in the solid are confirmed by the X-ray structural analysis of 1. In solution, the complexes 1 and 2 undergo an η1–η3–η1 rearrangement at 298 K interconverting the bonding site of the
CR2R3)(P–N–P′)]BF4 [R1 = R2 = R3 = H: (1); R1 = R2 = Ph, R3 = H: (2); R1 = R3 = H, R2 = Ph: (3); R1 = H, R2 = R3 = Me: (4)]. The P–N–P′ tridentate coordination and the η1-allyl bonding mode in the solid are confirmed by the X-ray structural analysis of 1. In solution, the complexes 1 and 2 undergo an η1–η3–η1 rearrangement at 298 K interconverting the bonding site of the ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2)(P–N–P′)]+ +48.78 kJ mol−1; [Pd(η1-CMe2–CH
CH2)(P–N–P′)]+ +48.78 kJ mol−1; [Pd(η1-CMe2–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2)(P–N–P′)]+ +69.35 kJ mol−1. The complexes react with
CH2)(P–N–P′)]+ +69.35 kJ mol−1. The complexes react with

 Please wait while we load your content...
Please wait while we load your content...