Isolation and molecular structures of novel organotellurium(iv) halides, oxyhalide and mixed halide†
Abstract
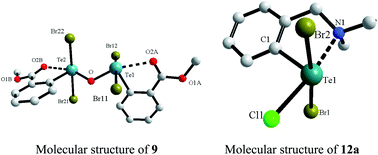
The formation of chiral (R)-[2-(4-ethyl-2-oxazolinyl)phenyl]tellurium(IV) trihalides (trichloride (5), tribromide (6) and triiodide (7)) and

 Please wait while we load your content...
Please wait while we load your content...