Phenyl substituted indenylphosphine ruthenium complexes as catalysts for dehydrogenation of alcohols†
Abstract
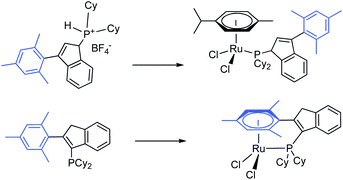
Thermal treatment of (1H-inden-3-yl)dicyclohexylphosphinium tetrafluoroborate (1) and (3-mesityl-1H-inden-3-yl)dicyclohexylphosphinium tetrafluoroborate (3) with tBuONa followed by [(η6-cymene)RuCl2)]2 in

 Please wait while we load your content...
Please wait while we load your content...