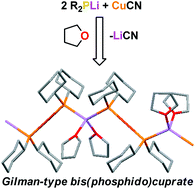
Reaction of in situ generated lithium phosphides with 0.5 eq. Cu(I) is employed as a means of targeting lithium phosphidocuprates of either Gilman- or Lipshutz-type formulation – e.g., (R2P)2CuLi·n(LiX) (n = 0, 1). For R = Ph, X = CN in toluene followed by thf or R = Ph, X = I in thf/toluene an unexpected product results. [(Ph2P)6Cu4][Li·4thf]21 reveals an ion-separated structure in the solid state, with solvated lithium cations countering the charge on an adamantyl dianion [(Ph2P)6Cu4]2−. Deployment of R = Ph, X = CN in thf affords a novel network based on the dimer of Ph2PCu(CN)Li·2thf 2 with trianions based on 6-membered (PCu)3 rings acting as nodes in the supramolecular array and solvated alkali metal counter-ions completing the linkers. Cy2PLi (Cy = cyclohexyl) has been reacted with CuCN in thf/toluene to yield Gilman-type lithium bis(phosphido)cuprate (Cy2P)2CuLi·2thf 3 by the exclusion of in situ generated LiCN. A polymer is noted in the solid state.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...