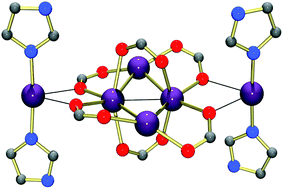
[Ag2(9-aca)2] (1) (9-acaH = 9-anthracenecarboxylic acid) reacts with a series of imidazoles to give [Ag(imidH)2.3(CH3CN)0.7](9-aca) (3), [Ag6(imidH)4(9-aca)6(MeOH)2] (4), {[Ag(1-Me-imid)2]2[Ag4(9-aca)6]} (5), {[Ag(1-Bu-imid)2]2[Ag4(9-aca)6]} (6) and [Ag(apim)](9-aca)·H2O (7) (imidH = imidazole; 1-Me-imid = 1-methylimidazole; 1-Bu-imid = 1-butylimidazole; apim = 1-(3-aminopropyl)imidazole). The mononuclear complex 3, hexanuclear 4–6, and polymeric 7, were all characterised using X-ray crystallography. While many of the complexes possess excellent in vitro antifungal and antibacterial activities they are, unanimously, more effective against fungal cells. The insect, Galleria mellonella, can survive high doses of the Ag(I) complexes administered in vivo, and a number of the complexes offer significant protection to larvae infected with a lethal dose of pathogenic Candida albicans cells.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...