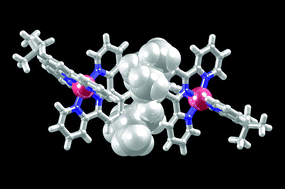
We describe the synthesis and characterization of 4′-tert-butyl-2,2′:6′,2′′-terpyridine (4′-tButpy, 1), a convergent tpy ligand that exhibits both a sterically demanding and solubilizing 4′-substituent. In the solid state, molecules of 1 pack with alternating tpy and tert-butyl domains, and the bulky alkyl substituents prevent the molecules from engaging in the face-to-face π-interactions which are typical of simple tpy ligands. Instead, the predominant packing forces involve CH⋯N hydrogen bonds and weak CH⋯π contacts. The syntheses of the homoleptic complexes [M(1)2][PF6]2 (M = Fe, Co, Zn and Ru) and the heteroleptic [Ru(tpy)(1)][PF6]2 are described. The complexes have been fully characterized in solution, including the 1H NMR spectroscopic characterization of the paramagnetic [Co(1)2][PF6]2. Cyclic voltammetric data are consistent with the tert-butyl substituent being slightly electron releasing. The single crystal structures of [Zn(1)2][PF6]2 and [Ru(1)2][PF6]2 have been determined; the compounds are essentially isomorphous. The packing of the cations is such that the tert-butyl substituents are accommodated in pockets between the tpy domains of adjacent cations, and as a consequence, the {M(tpy)2}-embrace that is a ubiquitous feature of many related structures is not observed.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...