Crystal and electronic structure, lattice dynamics and thermal properties of Ag(i)(SO3)R (R = F, CF3) Lewis acids in the solid state†
Abstract
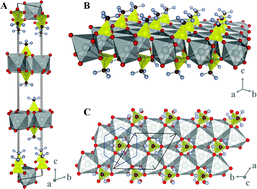
Trifluoromethansulfonate of silver(I), AgSO3CF3 (abbreviated AgOTf), extensively used in organic chemistry, and its fluorosulfate homologue, AgSO3F, have been structurally characterized for the first time. The crystal structures of both homologues differ substantially from each other. AgOTf crystallizes in a hexagonal system (R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) space group, No.148) with a = b = 5.312(3) Å and c = 32.66(2) Å, while AgSO3F crystallizes in a monoclinic system in the centrosymmetric P21/m space group (No.11) with a = 5.4128(10) Å, b = 8.1739(14) Å, c = 7.5436(17) Å, and β = 94.599(18)°, adopting a unique structure type (100 K data). There are two types of fluorosulfate anions in the structure; in one type the F atom is engaged in chemical bonding to Ag(I) and in the other type the F atom is terminal; accordingly, two resonances are seen in the 19F NMR spectrum of AgSO3F. Theoretical analysis of the electronic band structure and electronic density of states, as well as assignment of the mid- and far-infrared absorption and Raman scattering
space group, No.148) with a = b = 5.312(3) Å and c = 32.66(2) Å, while AgSO3F crystallizes in a monoclinic system in the centrosymmetric P21/m space group (No.11) with a = 5.4128(10) Å, b = 8.1739(14) Å, c = 7.5436(17) Å, and β = 94.599(18)°, adopting a unique structure type (100 K data). There are two types of fluorosulfate anions in the structure; in one type the F atom is engaged in chemical bonding to Ag(I) and in the other type the F atom is terminal; accordingly, two resonances are seen in the 19F NMR spectrum of AgSO3F. Theoretical analysis of the electronic band structure and electronic density of states, as well as assignment of the mid- and far-infrared absorption and Raman scattering

 Please wait while we load your content...
Please wait while we load your content...