Fabrication of magnetic mesoporous manganese ferrite nanocomposites as efficient catalyst for degradation of dye pollutants
Abstract
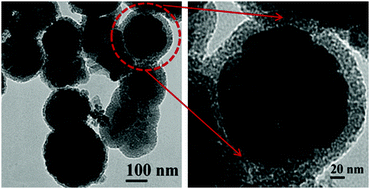
In this study, mesoporous silica encapsulated with magnetic MnFe2O4 nanoparticles is synthesized by a solvothermal method. The synthetic route is feasible and widely applicable. The obtained products have been characterized by an X-ray powder diffraction (XRD) pattern, field emission scanning electron microscopy (FESEM), transmission electron microscopy (TEM), high-resolution TEM (HRTEM) and nitrogen adsorption–desorption isotherm measurements. The synthesized magnetic mesoporous MnFe2O4 nanoparticles are monodispersed with a mean diameter of 200 nm, and have an obvious mesoporous silica shell of ∼20 nm. The surface area of magnetic mesoporous MnFe2O4 nanocomposites is 423 m2 g−1. The nanoparticles are superparamagnetic in nature at room temperature and can be separated by an external magnetic field. This magnetic mesoporous material is used as a catalyst for the degradation of methyl orange dye. The merits of the effect under different conditions like pH, temperature, light and sonolysis have been evaluated by investigating the degradation of azo dye. The mesoporous MnFe2O4 nanocomposites have effective adsorption of dyes inside the porous network followed by degradation with the central magnetite core and regeneration of the catalyst with the help of a simple magnet for successive uses.

 Please wait while we load your content...
Please wait while we load your content...