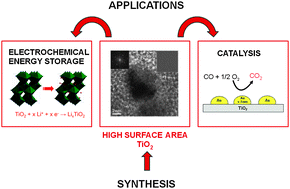
Titanium dioxide is one of the most intensely studied oxides due to its interesting electrochemical and photocatalytic properties and it is widely applied, for example in photocatalysis, electrochemical energy storage, in white pigments, as support in catalysis, etc. Common synthesis methods of titanium dioxide typically require a high temperature step to crystallize the amorphous material into one of the polymorphs of titania, e.g. anatase, brookite and rutile, thus resulting in larger particles and mostly non-porous materials. Only recently, low temperature solution-based protocols gave access to crystalline titania with higher degree of control over the formed polymorph and its intra- or interparticle porosity. The present work critically reviews the formation of crystalline nanoscale titania particles via solution-based approaches without thermal treatment, with special focus on the resulting polymorphs, crystal morphology, surface area, and particle dimensions. Special emphasis is given to sol–gel processes via glycolated precursor molecules as well as the miniemulsion technique. The functional properties of these materials and the differences to chemically identical, non-porous materials are illustrated using heterogeneous catalysis and electrochemical energy storage (battery materials) as example.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...