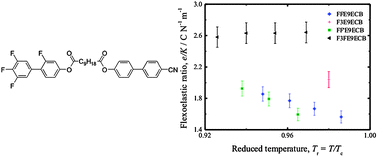
We present experimental results on the bulk flexoelectric coefficients e and effective elastic coefficients K of non-symmetric bimesogenic liquid crystals when the number of terminal and lateral fluoro substituents is increased. These coefficients are of importance because the flexoelastic ratio e/K governs the magnitude of flexoelectro-optic switching in chiral nematic liquid crystals. The study is carried out for two different types of linkage in the flexible spacer chain that connects the separate mesogenic units: these are either an ether or an ester unit. It is found that increasing the number of fluorine atoms on the mesogenic units typically leads to a small increase in e and a decrease in K, resulting in an enhancement of e/K. The most dramatic increase in e/K, however, is observed when the linking group is changed from ether to ester units, which can largely be attributed to an increase in e. Increasing the number of fluorine atoms does, however, increase the viscoelastic ratio and therefore leads to a concomitant increase in the response time. This is observed for both types of linkage, although the ester-linked compounds exhibit smaller viscoelastic ratios compared with their ether-linked counterparts. Highly fluorinated ester-linked compounds are also found to exhibit lower transition temperatures and dielectric anisotropies. As a result, these compounds are promising materials for use in electro-optic devices.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...