Are single C60fullerenes dielectric or metallic?
Abstract
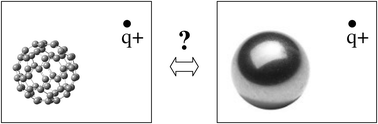
We present analytical solutions for the interaction energies between a static point charge and a metal- or a dielectric sphere. These solutions include polarization effects to infinite orders in the inverse of the distance between the point charge and the spheres. Further, we present Density Functional Theory (DFT) calculations of interaction energies for a point charge in ranges of fixed positions outside a neutral or a singly charged C60 molecule. Based on these DFT results, we conclude that the metal sphere model describes the electronic response of the C60 molecule much better than the dielectric sphere model. These findings are particularly important for calculations of energy barriers for charge transfer in reactions involving C60 molecules. The metal- and dielectric models should further be useful for descriptions of, for example, the polarizabilities of and interactions with conducting and insulating spherical clusters or particles.

 Please wait while we load your content...
Please wait while we load your content...