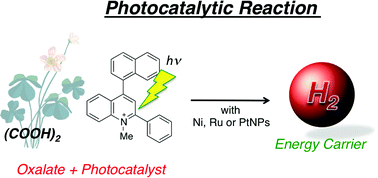
Photocatalytic hydrogen evolution has been made possible by using oxalate as a carbon-neutral electron source, metal nanoparticles as hydrogen-evolution catalysts and the 2-phenyl-4-(1-naphthyl)quinolinium ion (QuPh+–NA), which forms the long-lived electron-transfer state upon photoexcitation, as a photocatalyst. The hydrogen evolution was conducted in a deaerated mixed solution of an aqueous buffer and acetonitrile (MeCN) [1 : 1 (v/v)] by photoirradiation (λ > 340 nm). The gas evolved during the photocatalytic reaction contained H2 and CO2 in a molar ratio of 1 : 2, indicating that oxalate acts as a two-electron donor. The hydrogen yield based on the amount of oxalate reached more than 80% under pH conditions higher than 6. Ni and Ru nanoparticles as well as Pt nanoparticles act as efficient hydrogen-evolution catalysts in the photocatalytic hydrogen evolution. The photocatalyst for hydrogen evolution can be used several times without significant deactivation of the catalytic activity. Nanosecond laser flash photolysis measurements have revealed that electron transfer from oxalate to the photogenerated QuPh˙–NA˙+, which forms a π-dimer radical cation with QuPh+−NA [(QuPh˙–NA˙+)(QuPh+–NA)], occurs followed by subsequent electron transfer from QuPh˙–NA to the hydrogen-evolution catalyst in the photocatalytic hydrogen evolution. Oxalate acts as an efficient electron source under a wide range of reaction conditions.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...