The bulk and the gas phase of 1-ethyl-3-methylimidazolium ethylsulfate: dispersion interaction makes the difference†
Abstract
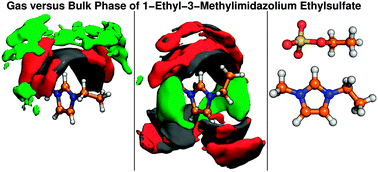
Ab initio molecular dynamics simulations were carried out on systems representing the gas and the bulk phase of 1-ethyl-3-methylimidazolium ethylsulfate [C2C1im][C2SO4]. The power

 Please wait while we load your content...
Please wait while we load your content...