Aqueous solutions of ionenes: interactions and counterion specific effects as seen by neutron scattering
Abstract
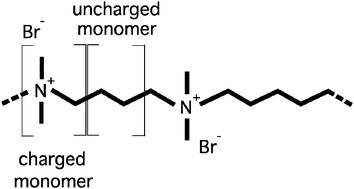
Aqueous solutions of ionenes with bromide and fluoride counterions have been investigated using small angle neutron scattering for the first time. Ionenes are a class of cationic polyelectrolytes based on quaternary ammonium atoms and, considering the very low solubility of their uncharged part (hydrocarbon chain), would be formally classified as hydrophobic. Ionenes present important structural differences over previously studied polyelectrolytes: (a) charge is located on the polyelectrolyte backbone, (b) the distance between charges is regular and tunable by synthesis, (c) hydrophobicity comes from methylene groups of the backbone and not from bulky side groups. Results for Br ionenes feature a disappearance of the well-known polyelectrolyte peak beyond a given monomer concentration. Below this concentration, the position of the peak depends on the chain charge density, fchem, and scales as fchem0.30±0.04. This is an indication of a hydrophilic character of the ionene backbone. In addition, osmotic coefficients of ionene solutions resemble again other hydrophilic polyelectrolytes, featuring no unusual increase in the water activity (or a significant counterion condensation). We conclude that despite the hydrophobicity of the hydrocarbon chain separating charged centers on ionenes, these chains behave as hydrophilic. In contrast to Br ionenes, the polyelectrolyte peak remains at all concentrations studied for the single F

 Please wait while we load your content...
Please wait while we load your content...