Aerobic oxidation of β-isophorone catalyzed by N-hydroxyphthalimide: the key features and mechanism elucidated†
Abstract
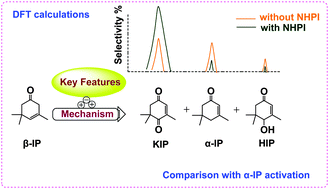
Due to the insufficient understanding of the selective oxidation mechanism of α/β-isophorones (α/β-IP) to ketoisophorone (KIP), the key features in the β-IP oxidation catalyzed by N-hydroxyphthalimide (NHPI) have been explored via theoretical calculations. β-IP is more favourable to being activated by phthalimide-N-oxyl radical (PINO˙) and peroxyl radical (ROO˙) than α-IP owing to the different C–H strengths at their reactive sites, thereby exhibiting selective product distributions. It was found that NHPI accelerates β-IP activation due to the higher reactivity of PINO˙ than ROO˙ and the equilibrium reaction between them, yielding considerable hydroperoxide (ROOH) and ROO˙. In addition, the ROOH decomposition is more favourable via α-H abstraction by radicals than its self-dehydration and thermal dissociation. The strong exothermicity of this α-H abstraction, along with that from H-abstraction by co-yielded hot HO˙, is in favor of the straightforward formation of KIP, simultaneously leading to the isomerization of a few β-IP to α-IP and production of 4-hydroxyisophorone (HIP) and water. The proposed mechanisms, consistent with the experimental observations, allow for the deeper understanding and effective design of oxidation systems involving similar substrates or NHPI analogues that are of industrial importance.

 Please wait while we load your content...
Please wait while we load your content...