The entrance complex, transition state, and exit complex for the F + H2O → HF + OH reaction. Definitive predictions. Comparison with popular density functional methods
Abstract
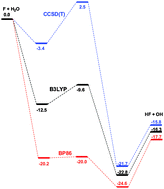
Following the H + H2 and F + H2 reactions, the fluorine atom – water system has the potential to become one of the best understood chemical reactions. Stationary points for the F +

 Please wait while we load your content...
Please wait while we load your content...