Solution and nanoscale structure selection: implications for the crystal energy landscape of tetrolic acid†
Abstract
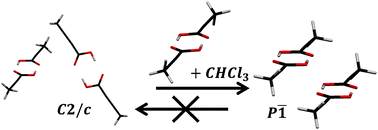
Solution and growth effects are in many cases critical in determining which crystal structure (polymorph) a molecule will adopt. Contemporary crystal structure prediction (

 Please wait while we load your content...
Please wait while we load your content...