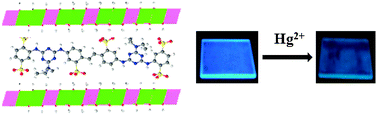
Ordered ultrathin films (UTFs) with blue luminescence based on a styrylbiphenyl derivative (BTBS) and Mg–Al-layered double hydroxide (LDH) nanosheets have been constructed employing the layer-by-layer assembly technique. UV-visible absorption and fluorescence spectroscopy showed a stepwise and regular growth of the films upon increasing the number of deposition cycles. XRD, AFM and SEM indicated that the films possess a periodic layered structure with a period of ca. 1.5 nm, and uniform surface morphology. The film thickness can be precisely controlled in the range ca. 15–53 nm. The BTBS–LDH UTFs exhibit improved UV-light resistance capability compared with the pristine BTBS and show well-defined polarized photoemission, with anisotropy of ca. 0.24. The UTFs show a fast, selective and reversible luminescent response to aqueous solutions containing different heavy metal ions, with the most significant luminescent quenching occurring for the Hg2+ solution, shedding light on the fact that these films can serve as a new type of selective solid luminescent metal-ion sensor.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...