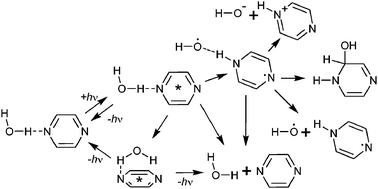
A unified picture is presented of water interacting with pyridine, pyridazine, pyrimidine, and pyrazine on the S1 manifold in both gas-phase dimers and in aqueous solution. As (n,π*) excitation to the S1 state removes electrons from the ground-state hydrogen bond, this analysis provides fundamental understanding of excited-state hydrogen bonding. Traditional interpretations view the excitation as simply breaking hydrogen bonds to form dissociated molecular products, but reactive processes such as photohydrolysis and excited-state proton coupled electron transfer (PCET) are also possible. Here we review studies performed using equations-of-motion coupled-cluster theory (EOM-CCSD), multireference perturbation theory (CASPT2), time-dependent density-functional theory (TD-DFT), and excited-state Monte Carlo liquid simulations, adding new results from symmetry-adapted-cluster configuration interaction (SAC-CI) and TD-DFT calculations. Invariably, gas-phase molecular dimers are identified as stable local minima on the S1 surface with energies less than those for dissociated molecular products. Lower-energy biradical PCET minima are also identified that could lead to ground-state recombination and hence molecular dissociation, dissociation into radicals or ions, or hydration reactions leading to ring cleavage. For pyridine.water, the calculated barriers to PCET are low, suggesting that this mechanism is responsible for fluorescence quenching of pyridine.water at low energies rather than accepted higher-energy Dewar-benzene based “channel three” process. Owing to (n,π*) excitation localization, much higher reaction barriers are predicted for the diazines, facilitating fluorescence in aqueous solution and predicting that the as yet unobserved fluorescence from pyridazine.water and pyrimidine.water should be observable. Liquid simulations based on the assumption that the solvent equilibrates on the fluorescence timescale quantitatively reproduce the observed spectral properties, with the degree of (n,π*) delocalization providing a critical controlling factor.

 Please wait while we load your content...
Please wait while we load your content...