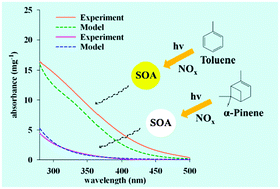
A new model for predicting the UV-visible absorption spectra of secondary organic aerosols (SOA) has been developed. The model consists of two primary parts: a SOA formation model and a semiempirical quantum chemistry method. The mass of SOA is predicted using the PHRCSOA (Partitioning Heterogeneous Reaction Consortium Secondary Organic Aerosol) model developed by Cao and Jang [Environ. Sci. Technol., 2010, 44, 727]. The chemical composition is estimated using a combination of the kinetic model (MCM) and the PHRCSOA model. The absorption spectrum is obtained by taking the sum of the spectrum of each SOA product calculated using a semiempirical NDDO (Neglect of Diatomic Differential Overlap)-based method. SOA was generated from the photochemical reaction of toluene or α-pinene at different NOx levels (low NOx: 24–26 ppm, middle NOx: 49 ppb, high NOx: 104–105 ppb) using a 2 m3 indoor Teflon film chamber. The model simulation reasonably agrees with the measured absorption spectra of α-pinene SOA but underestimates toluene SOA under high and middle NOx conditions. The absorption spectrum of toluene SOA is moderately enhanced with increasing NOx concentrations, while that of α-pinene SOA is not affected. Both measured and calculated UV-visible spectra show that the light absorption of toluene SOA is much stronger than that of α-pinene SOA.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...