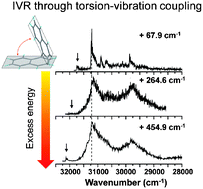
The role of ring torsion in the enhancement of intramolecular vibrational energy redistribution (IVR) in aromatic molecules was investigated by conducting excitation and dispersed fluorescence spectroscopy of 1,1′-binaphthyl (1,1′-BN) and 2,2′-BN. The dispersed fluorescence spectra of 1,1′-BN in the origin region of S1–S0 were well resolved, which presented 25–27 cm−1 gaps of torsional mode in the ground state. The overall profile of the dispersed spectra of 1,1′-BN is similar to that of naphthalene. In contrast, the spectra of 2,2′-BN were not resolved due to the multitude of the active torsional modes. In both cases, dissipative IVR was observed to take place with a relatively small excess vibrational energy: 237.5 cm−1 for 1,1′-BN and 658 cm−1 for 2,2′-BN, which clearly shows that ring torsion efficiently enhances the IVR rate. Ab initio and density functional theory calculations with medium-sized basis sets showed that the torsional potential of 1,1′-BN has a very flat minimum over the range of torsional angles from ca. 60° to 120°, whereas that of 2,2′-BN showed two well-defined potential minima at ca. 40° and 140°, in resemblance to the case of biphenyl. In this work, we propose that aromatic molecules be classified into “strong” and “weak” torsional hindrance cases: molecules with strong hindrance case show shorter torsional progressions and more effective IVR dynamics than do those with weak hindrance.

 Please wait while we load your content...
Please wait while we load your content...