Nature of the attractive interaction between proton acceptors and organic ring systems†
Abstract
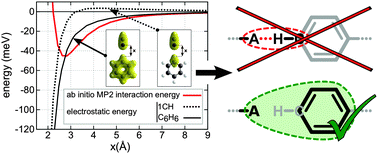
Systematic ab initio calculations are combined with a deconvolution of electrostatic contributions to analyze the interplay between potential hydrogen bond acceptors and organic rings with Csp2–H groups (

 Please wait while we load your content...
Please wait while we load your content...