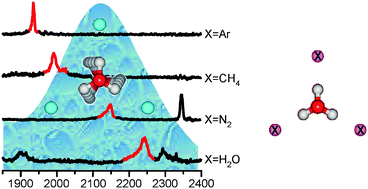
The harmonic approximation provides a powerful approach for interpreting vibrational spectra. In this treatment, the energy and intensity of the 3N − 6 normal modes are calculated using a quadratic expansion of the potential energy and a linear expansion of the dipole moment surfaces, respectively. In reality, transitions are often observed that are not accounted for by this approach (e.g. combination bands, overtones, etc.), and these transitions arise from inherent anharmonicities present in the system. One interesting example occurs in the vibrational spectrum of H2O(l), where a band is observed near 2000 cm−1 that is commonly referred to as the “association band”. This band lies far from the expected bend and stretching modes of the water molecule, and is not recovered at the harmonic level. In a recent study, we identified a band in this spectral region in gas-phase clusters involving atomic and molecular adducts to the H3O+ ion. In the current study we probe the origins of this band through a systematic analysis of the argon-predissociation spectra of H3O+·X3 where X = Ar, CH4, N2 or H2O, with particular attention to the contributions from the non-linearities in the dipole surfaces, often referred to as non-Condon effects. The spectra of the H3O+ clusters all display strong transitions between 1900–2100 cm−1, and theoretical modeling indicates that they can be assigned to a combination band involving the HOH bend and frustrated rotation of H3O+ in the solvent cage. This transition derives its oscillator strength entirely from strong non-Condon effects, and we discuss its possible relationship to the association band in the spectrum of liquid water.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...