The unexpected but predictable tetrazole packing in flexible 1-benzyl-1H-tetrazole†
Abstract
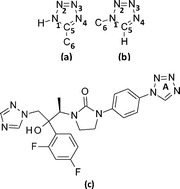
The crystal structure of 1-benzyl-1H-tetrazole, C8H8N4, was undertaken to study the geometry and intermolecular interactions of the (1-substituted) 1H-tetrazole moiety. It crystallizes in the monoclinic space group P21 with unit cell dimensions a = 7.6843(5), b = 5.5794(4), c = 9.4459(7) Å, β = 100.949(4)°, V = 397.61(5) Å3, Z = 2, density (calculated) = 1.338 gcm−3. The packing of the molecules in the crystal structure is dominated by a number of weak intermolecular hydrogen bonds of the type CH⋯N and CH⋯C. This results in segregated infinite S-shaped layers of

 Please wait while we load your content...
Please wait while we load your content...