Polymorphism in co-crystals: a metastable form of the ionic co-crystal 2 HBz·1 NaBz crystallised by flash evaporation†
Abstract
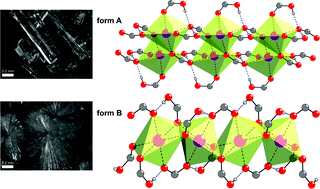
While polymorphism is a wide spread phenomenon, the number of reported polymorphic co-crystals is still very limited. Here we report the synthesis and structural characterisation of such a rare case of polymorphism with a co-crystal of benzoic acid (HBz) and sodium benzoate (NaBz). Flash evaporation yielded a new polymorph of this ionic co-crystal with a stoichiometry of 2 HBz·1 NaBz. The thermodynamic relationship between the known and the new polymorph was determined to be of enantiotropic nature. At room temperature, this new form B is metastable. While the known form A is composed of one dimensional tapes, form B is built from infinite rods. The coordination sphere of sodium, however, in both forms is octahedral and the packing around it is dense.

 Please wait while we load your content...
Please wait while we load your content...