Facile synthesis of uniform and well-defined single-crystal sodium tantalate cubes and their assembly into oriented two-dimensional nanofilm
Abstract
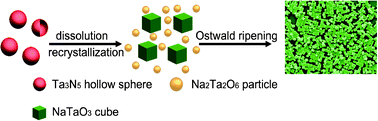
Uniform and well-defined single-crystal NaTaO3 cubes were successfully synthesized through a solvothermal method using monodisperse meso-microporous Ta3N5 hollow spheres as precursors. The structure and morphology of NaTaO3 cubes were investigated by

 Please wait while we load your content...
Please wait while we load your content...