Effect of increasing ligand length on the structure of silver complexes†
Abstract
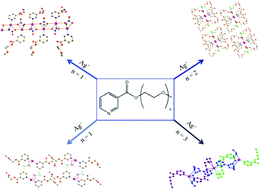
A new series of ligands in which nicotinic acid was linked to different monomethyloligoethers and their corresponding silver complexes were prepared. The crystal structures of the silver compounds were investigated to determine the effect of increasing flexibility of the ligands on coordination to the metal ion and crystal packing. We observed the formation of one-dimensional coordination polymers for the shortest ligand derived from monoethylene glycol, the formation of stacks for the diethylene-glycol-based ligand with silver triflate, and even a helical structure for the triethylene-glycol-derived ligand complex. The three ligands, ([C9H11O3N] (L1), [C11H15O4N] (L2), and [C13H19O5N] (L3)), and the four new complexes, [Ag(L1)2(NO3)] (1), [Ag(L1)2(SO3CF3)] (2), [(Ag(L2)2(SO3CF3)] (3), and [Ag(L3)2]PF6 (4)), are presented in this paper along with an analysis of their structural features.

 Please wait while we load your content...
Please wait while we load your content...