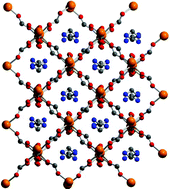
Two novel polymeric Mg formates [(Fmd)Mg(HCOO)3]∞ (1) and [(Gua)Mg(HCOO)3]∞ (2) containing the formamidinium [Fmd+, (NH2–CH+–NH2)] and guanidinium [Gua+, C+(NH2)3] cations have been prepared under solvothermal conditions. 1 and 2 are isostructural; they crystallize in the orthorhombic space group Pnna. Their 3D scaffolds consists of Mg ions in an octahedral coordination environment bridged by formate ligands. The overall framework charge is negative, and the cations are located in the centre of the lattice cavities, forming extensive N–H⋯O hydrogen bonding with the surrounding cage. Both compounds have been characterized through single-crystal and powder X-ray diffraction, 1D and 2D solid-state NMR (1H, 13C and 15N), IR spectroscopy and TG-MS analysis. Their chemical reactivity towards ion exchange and ability as CO2 storage materials has been finally examined.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...