Hierarchical flower-like Fe3O4 and γ–Fe2O3nanostructures: Synthesis, growth mechanism and photocatalytic properties†
Abstract
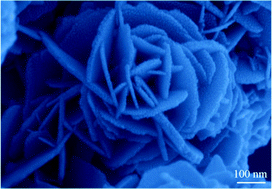
The present study describes a facile, economical and environment-friendly aqueous solution method of fabricating hierarchical flower-like Fe3O4

 Please wait while we load your content...
Please wait while we load your content...