Isostructurality in pharmaceutical salts: How often and how similar?
Abstract
Clarity around the intended definitions of isostructurality in solid forms is highlighted with respect to the
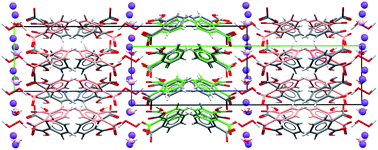
- This article is part of the themed collection: Crystal engineering and crystallography in the pharmaceutical industry

 Please wait while we load your content...
Please wait while we load your content...