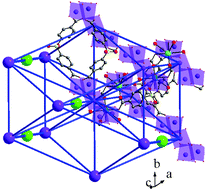
Four new three-dimensional Mn2+ ion-containing compounds have been prepared by employing a hydrothermal reaction between Mn(CH3COO)2·4H2O, sulfodibenzoic acid (H2SDBA), imidazole, alkali hydroxide and water at 220 °C for 1 day. The compounds have Mn5 (1–4) clusters connected by SDBA, forming the three-dimensional structure. A time and temperature dependent study on the synthesis mixture revealed the formation of a one-dimensional compound, Mn(SDBA)(H2O)2, at lower temperatures (T ≤ 180 °C). The stabilization of the fcu related topology in the compounds is noteworthy. Magnetic studies indicate strong anti-ferromagnetic interactions between the Mn2+ ions within the clusters in the temperature range 75–300 K. The rare participation of a sulfonyl group in the bonding is important and can pave way for the design of new structures.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...