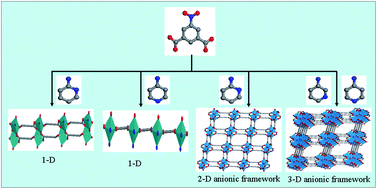
A series of cadmium(II) and zinc(II) metal–organic frameworks, [Cd(NIPH)(2-apy)(H2O)·(H2O)] (1), [Cd(NIPH)(4-apy)2(H2O)] (2), [Zn2(NIPH)2(μ3-OH)(H2O)·(2-Hapy)] (3), [Zn5(NIPH)4(μ3-OH)2(μ2-OH)(H2O)·(3-Hapy)] (4), and [Zn5(NIPH)4(μ3-OH)2(μ2-OH)(H2O)·(4-Hapy)·(H2O)] (5), were synthesized by hydrothermal reactions of the corresponding Cd(II) and Zn(II) salts with 5-nitroisophthalic acid (H2NIPH) in the presence of aminopyridine (apy) auxiliary ligands. These frameworks exhibit three typical structural features: 1 and 2 possess a 1-D double-stranded loop-like chain and a 1-D linear chain formed by [Cd2N2O10] dinuclear building blocks and [CdN2O5] monoclear building blocks, respectively; 3 is a 2-D anionic framework consisting of [Zn4O16] tetranuclear building blocks; both 4 and 5 are 3-D anionic frameworks constructed by both [Zn4O16] tetranuclear and [Zn6O22] hexanuclear building blocks. The apy ligands participate in constructing frameworks of 1 and 2, but are protonated in the channels of 3–5 as charge-balancing and space-filing agents. The thermal stability, luminescent properties and ion-exchange behavior of the complexes were investigated.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...