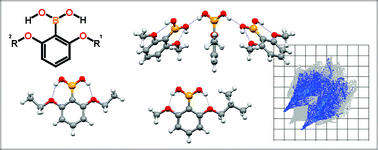
The structures of three ortho-alkoxyphenylboronic acids (2-methoxy-, 2-ethoxy-, 2-isobutoxy-), and three diortho-alkoxyphenylboronic acids (2,6-dimethoxy-, 2,6-diethoxy- and 2-isobutoxy-6-methoxy) were determined by single crystal X-ray diffraction. The study was undertaken with the intention of designing a novel boronic acid having a monomeric structure, which to date has been an unavailable building block for crystal engineering. This motif can be enhanced by involving two hydroxyl groups at the boron atom in intramolecular hydrogen bonds. Although monosubstituted systems form typical dimers in the crystal lattice, disubstituted species reveal a much bigger variety of possible interactions. Among the analyzed compounds, 2,6-dimethoxyphenylboronic acid and 2,6-diethoxyphenylboronic acid crystallize in two polymorphic forms each. The unprecedented packing with monomers as the dominant structural motif has been found in the crystal structure of 2-isobutoxy-6-methoxyphenylboronic acid and one of the polymorphs of both 2,6-dimethoxyphenylboronic acid and 2,6-diethoxyphenylboronic acid. This description of the molecular packing is also supported by the analysis of fingerprint 2-D plots based on the Hirshfeld surfaces. The variety of possible types of interactions either within a single moiety or between moieties in dimers were additionally analyzed on the basis of the interaction energies, which have been estimated by ab initio calculations at the MP2/6-31+G* and B3LYP/6-311+G** level of theory.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...