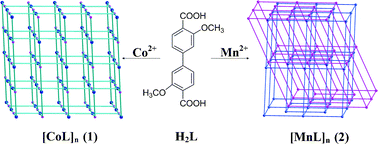
Two novel 3D metal–organic frameworks, [ML]n (M = Co, 1; Mn, 2) were successfully prepared in solvothermal conditions using 3,3′-dimethoxy-4,4′-biphenyldicarboxylic acid (H2L) as the ligand. X-Ray crystallography analysis reveals that MOF 1 crystallizes in the monoclinic system, space group P21/c in contrast to MOF 2 in the tetragonal system, space group I![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) . MOF 1 contains an elongated [CoO6] octahedron with two bound methoxy groups in the trans position, whereas MOF 2 has a compressed [MnO6] octahedron with two coordinated methoxy groups in the cis arrangement. The ligand L shows a novel bis(tridentate) bridging coordination mode. MOF 1 exhibits a 3D framework with CdSO4 (cds) topology consisting of two different nodes and good thermal stability (313 °C). MOF 2 is a doubly interpenetrated 3D α-Po framework with a higher thermal stability (368 °C). The study of magnetic properties in the temperature range of 1.8–300 K shows the occurrence of weak ferromagnetic interactions (J = 0.15 K) between the high-spin Co(II) ions in 1, but a weak antiferromagnetic coupling (J = −0.15 cm−1) between Mn(II) ions in 2 due to the syn-anti carboxylate bridge.
. MOF 1 contains an elongated [CoO6] octahedron with two bound methoxy groups in the trans position, whereas MOF 2 has a compressed [MnO6] octahedron with two coordinated methoxy groups in the cis arrangement. The ligand L shows a novel bis(tridentate) bridging coordination mode. MOF 1 exhibits a 3D framework with CdSO4 (cds) topology consisting of two different nodes and good thermal stability (313 °C). MOF 2 is a doubly interpenetrated 3D α-Po framework with a higher thermal stability (368 °C). The study of magnetic properties in the temperature range of 1.8–300 K shows the occurrence of weak ferromagnetic interactions (J = 0.15 K) between the high-spin Co(II) ions in 1, but a weak antiferromagnetic coupling (J = −0.15 cm−1) between Mn(II) ions in 2 due to the syn-anti carboxylate bridge.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) . MOF 1 contains an elongated [CoO6] octahedron with two bound methoxy groups in the trans position, whereas MOF 2 has a compressed [MnO6] octahedron with two coordinated methoxy groups in the cis arrangement. The
. MOF 1 contains an elongated [CoO6] octahedron with two bound methoxy groups in the trans position, whereas MOF 2 has a compressed [MnO6] octahedron with two coordinated methoxy groups in the cis arrangement. The 

 Please wait while we load your content...
Please wait while we load your content...