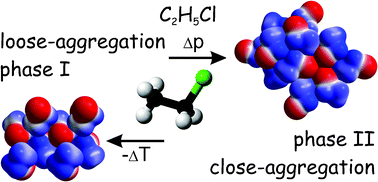
Low-density liquids and solids, with all intermolecular contacts longer than the sum of van der Waals radii, are formed by all ethanes chlorinated at one locant: CH2ClCH3, CHCl2CH3 and CCl3CH3. The concepts of molecular symmetry described by Carnelley and that of point groups have been compared. Carnelley's rule, when applied to liquid and solid chloroethanes clearly reveals the density dependence on the presence of intermolecular Cl⋯Cl and H⋯Cl short contacts, or their absence due to steric hindrances of overcrowded substituents. At 2.62 GPa, CH2ClCH3 freezes directly into phase II, with molecules arranged into layers with short Cl⋯Cl, H⋯Cl and H⋯H contacts. Only for CH2ClCH3, both the low-density phase at low temperature and closely-packed phase above 2.62 GPa have been observed.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...