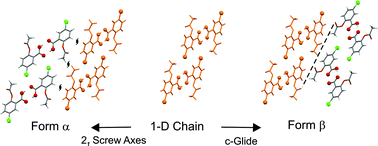
From an initial crystallographic study of a large family of ring-substituted 2-acetoxybenzoic acids, only one member - the 5-chloro derivative - showed polymorphism, with two forms (α and β) identified, the former having a marginally higher melting point. The two structures show a close 1-D similarity through a row of carboxylic dimers connected viaCl⋯O halogen bonds, which assemble to give an approximate 2-D similarity by the stacking of these rows in a second direction. However, the further packing arrangement of the resulting 2-D stacks is significantly different in the two forms, through different symmetry arrangements and subtle variations in C–H⋯O weak hydrogen bonds. A parallel crystal structure prediction (CSP) calculation identified the two experimental polymorphs in the correct stability order with effective energy rankings of 2 and 3 (lattice energy difference of 0.2 kcal mol−1). The lowest energy crystal structure found during the CSP, as yet not found experimentally, is more stable than the lowest energy observed polymorph by 0.08 kcal mol−1. The predicted forms mostly comprise pairs of structures with nearly identical crystal structure arrangements, which differ only in the positioning of the carboxylate protons in the common carboxylate-carboxylate hydrogen-bonded dimers, relative to the positions of the neighboring acetyl substituents (syn or anti). The CSP calculations identify the correct isomer for each polymorph. One of the additional predicted forms is found to have 3-D packing similarity, and others partial similarities, with the crystal structures of particular members of the extended aspirin family.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...