Lanthanide-pyridyl-2,5-dicarboxylate N-oxide frameworks with rutile topology†
Abstract
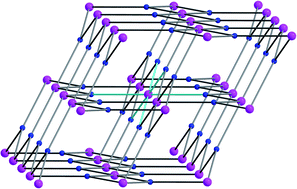
Four novel lanthanide-organic frameworks Ln(2,5-pdco)(CH3COO)(H2O) [Ln = La (1), Eu (2), Gd (3), Tb (4); 2,5-H2pdco = pyridyl-2,5-dicarboxylic acid N-oxide] have been synthesized and characterized. The coordination networks are similarly constructed viaLn2(μ-CH3COO)2 dinuclear secondary building units (SBUs), which are further linked by six 3-connected 2,5-pdco tectons to result in a novel 3D (3,6)-connected framework with

 Please wait while we load your content...
Please wait while we load your content...