Flow synthesis of arylboronic esters bearing electrophilic functional groups and space integration with Suzuki–Miyaura coupling without intentionally added base†
Abstract
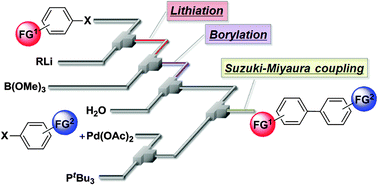
We found that an integrated flow microreactor system enables the preparation of boronic esters bearing electrophilic functional groups using organolithium chemistry and that it allows for their use in Suzuki–Miyaura cross-coupling without intentionally added base. Based on this method, cross-coupling of two aryl halides bearing electrophilic functional groups was accomplished to obtain the corresponding

 Please wait while we load your content...
Please wait while we load your content...