Verification of stereospecific dyotropic racemisation of enantiopure d and l-1,2-dibromo-1,2-diphenylethane in non-polar media†
Abstract
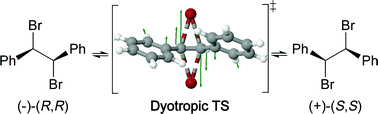
The first example of a dyotropic rearrangement of an enantiomerically pure, conformationally unconstrained, vicinal dibromide confirms theoretical predictions: D and L-1,2-dibromo-1,2-diphenylethane racemise stereospecifically in refluxing

 Please wait while we load your content...
Please wait while we load your content...