Desymmetrization of cyclohexadienones viad-camphor-derived triazolium salt catalyzed intramolecular Stetter reaction†
Abstract
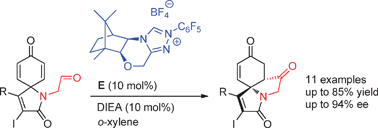
Enantioselective desymmetrization of cyclohexadienones via a D-camphor-derived triazolium salt catalyzed intramolecular Stetter reaction was realized. With 10 mol% of

 Please wait while we load your content...
Please wait while we load your content...