Intermolecular central to axial chirality transfer in the self-assembled biphenyl containing amino acid–oxalamide gelators†
Abstract
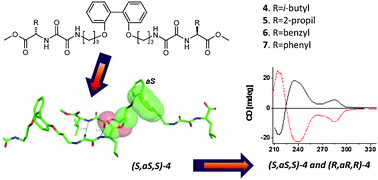
Chiral amino acid and biphenyl incorporating oxalamide gelators 4–7 with large, 9 bond distance between chiral centres and biphenyl units have been studied. CD investigation of 4-octanol gel and the crystal structure of rac-4 reveal that efficient central to axial chirality transfer occurs by intermolecular interactions in gel and solid state assemblies.

 Please wait while we load your content...
Please wait while we load your content...