Convenient method for the functionalization of the 4- and 6-positions of the androgen skeleton†
Abstract
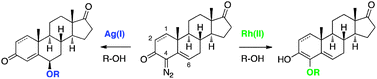
The preparation and reactivity of steroidal vinyldiazo compounds is reported, providing a convenient, substituent tolerant, chemo- and stereoselective entry into 4- and 6-substituted

 Please wait while we load your content...
Please wait while we load your content...