The formal potentials and electrode kinetics of the proton/hydrogen couple in various room temperature ionic liquids†
Abstract
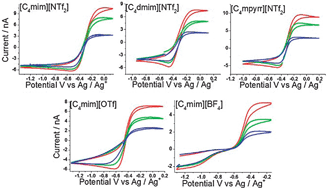
The Hydrogen evolution reaction has been quantitatively investigated at a Pt electrode in series of room temperature
- This article is part of the themed collection: Ionic Liquids

 Please wait while we load your content...
Please wait while we load your content...