Regioselective opening of proximally sulfato-capped cyclodextrins†
Abstract
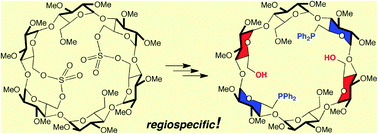
Sulfato groups capping one or two pairs of adjacent glucose units in methylated
* Corresponding authors
a
Laboratoire de Chimie Inorganique Moléculaire et Catalyse, Institut de Chimie UMR 7177 CNRS, Université de Strasbourg, 67008 Strasbourg Cedex, France
E-mail:
d.armspach@unistra.fr, dmatt@unistra.fr
Fax: +333 68 851 637
b Institut de Physique de Rennes, UMR 6251 CNRS, Université de Rennes 1, Campus de Beaulieu – Bâtiment 11A, 35042 Rennes cedex, France
Sulfato groups capping one or two pairs of adjacent glucose units in methylated

 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
M. Jouffroy, R. Gramage-Doria, D. Armspach, D. Matt and L. Toupet, Chem. Commun., 2012, 48, 6028 DOI: 10.1039/C2CC31302B
To request permission to reproduce material from this article, please go to the Copyright Clearance Center request page.
If you are an author contributing to an RSC publication, you do not need to request permission provided correct acknowledgement is given.
If you are the author of this article, you do not need to request permission to reproduce figures and diagrams provided correct acknowledgement is given. If you want to reproduce the whole article in a third-party publication (excluding your thesis/dissertation for which permission is not required) please go to the Copyright Clearance Center request page.
Read more about how to correctly acknowledge RSC content.
 Fetching data from CrossRef.
Fetching data from CrossRef.
This may take some time to load.
Loading related content
